Important Compounds of Group 17 Elements
Important Compounds of Group 17 Elements: Overview
This topic covers concepts, such as, Important Compounds of Group 17 Elements, Chlorine, Oxoacids of Halogens & Structures of Oxoacids of Chlorine etc.
Important Questions on Important Compounds of Group 17 Elements
The products obtained from the following chemical reactions are respectively
(i)
(ii)
What products are expected from the disproportionation reaction of hypochlorous acid?
Given below are two statements : one is labelled as Assertion and the other is labelled as Reason .
Assertion : is more reactive than .
Reason : bond is weaker than bond. In the light of the above statements, choose the most appropriate answer from the options given below: (1) Both and are correct but is not the correct explanation of .
This question concerns the elements of group fluorine, chlorine, bromine and iodine. When sodium chloride is treated with concentrated sulphuric acid, a colourless gas, which fumes in moist air, is formed. When sodium iodide is treated in the same way a coloured vapour, is product. If phosphoric acid is used instead of sulphuric acid, a colourless gas is produced in each reaction.
Behaviour of and is different towards because:
This question concerns the elements of group fluorine, chlorine, bromine and iodine. When sodium chloride is treated with concentrated sulphuric acid, a colourless gas, which fumes in moist air, is formed. When sodium iodide is treated in the same way a coloured vapour, is formed as a product. Gases and are respectively
What is the colour of chlorine gas.
What is the name of the given reaction.
The anhydride of the oxoacid is
The least number of oxyacids are formed by:
In the laboratory preparation of hydrochloric acid, a funnel arrangement is used to prepare for the following reason:
Which gas is evolved when hydrochloric acid is added to Manganese oxide?
Identify the chemical formula of oxoacid of halogen, chloric acid.
The correct order of acidity of oxoacids of chlorine is
Identify the name of oxoacid of halogen, .
Which oxoacid of chlorine is the most acidic?
Arrange the following :
Increasing order of thermal stability
Increasing acid strength
Increasing reducing nature
Increasing oxidation number of iodine
Increasing acid strength
Increasing oxidising power
Increasing acid strength
Increasing electronegativity
Which of the following inter halogen compounds is impossible to exist
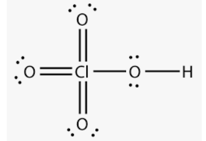
Above structure is of _____ acid.
Which of the following are correct for :
When chlorine is treated with dry slaked lime, the product formed will be: